The requirements for corrosion prevention and control should be considered at all stages of the design process. The aim should be to ensure that the final structure will allow manufacture, assembly and in service and maintenance to be performed as easily as possible, in a manner that will maintain the quality of the structure and its ability to resist corrosion.
4.4.4.1. Corrosion Prevention By Geometric Design
The most important way to minimize the potential for corrosion by large scale geometric design is to provide adequate drain paths and avoid regions where water can collect.
The examples below are for metal structure, the same principals should be applied to composite structure.

 AGARD-AG-278, 1985)
AGARD-AG-278, 1985) 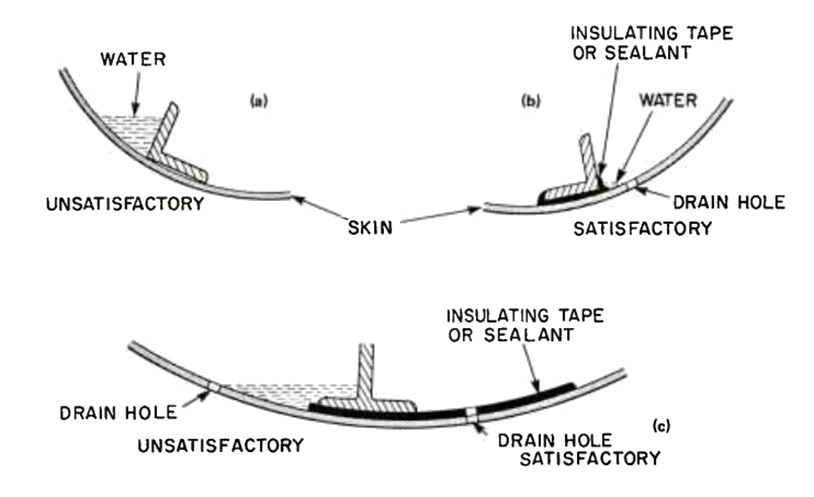
 AGARD-AG-278, 1985)
AGARD-AG-278, 1985) It is important that large assemblies are created assuming that condensation can occur on any surface. Assemblies must have managed drain paths that lead to drain holes or drain valves to avoid regions where moisture can collect and the corrosion problem this can create.
4.4.4.2. Corrosion Prevention by Surface Treatments and Protective Coatings
Exposed surfaces of the aircraft can be modified chemically to render them less susceptible to corrosion. Surfaces can also be protected with a coating or paint. Some processes (cladding) are applied at the mill stage by the manufacturer. Anodizing, chromate filming or plating are applied by the aircraft OEM after the part has been formed. Surface pre-treatments by chemical or mechanical means are also important in creating good adhesion for paint to the surface.
Cladding
May high strength aluminum alloys are susceptible to corrosion. Corrosion resistance can be improved by metallurgically bonding to the core alloy a surface layer of pure aluminum or an alloy with good corrosion resistance. The cladding should be anodic to the core metal so if the cladding becomes damaged by or the core alloy is exposed the cladding will provide cathodic protection.The thickness of the cladding is usually between 2% and 5% of the total sheet or plate thickness and the cladding is usually a softer or lower strength alloy. This can affect fatigue strength, abrasion resistance and buckling performance. The effect of cladding on buckling performance can be estimated using the method in section 15.2.9 of this book.
Surface Conversion Coatings
Surface conversion coatings are produced by chemical action with electrical assistance. The treatment changes the immediate surface of metal into a film of metallic oxide or compound which has greater corrosion resistance than the base metal. Surface conversion coatings also offer a good base for paint.
Anodizing: The electrolytic oxidation of a surface to produce a tightly adherent oxide scale which is thicker than the naturally occurring film. The Anodized surface is hard and abrasion resistant. Anodizing alone cannot be relied upon to provide effective corrosion resistance to corrosion prone alloys, further protection by painting is required. Anodic coatings breakdown in highly alkaline (pH > 8.5) and highly acid (pH < 4.0) solution. Anodic coatings are relatively brittle and may crack under stress, supplementary protection such as painting is particularly important with stress corrosion prone alloys. Anodic coatings are used with aluminum, magnesium and titanium alloys
Chromate Filming: Proprietary chromate filmings are available for aluminum, magnesium, cadmium and zinc alloys. Chromate films are usually about 5µm thick. The film contains soluble chromates which act as corrosion inhibitors and they provide a modest improvement in corrosion resistance of the base metal.Their main purpose is the provide suitable surface for sealing resins or paint.
Phosphate Coating: Several phosphate coatings have been developed for use on steel. The coatings consist of a thick porous layer of fine phosphate crystals tightly bonded to the steel. The coating does not provide effective corrosion resistance when used alone, but the coating provides an excellent base for oils waxes or paints and the help to prevent the spreading of rust under layers of paint. Phosphating is not widely used in aircraft structures.
Nitriding: Steels containing nitride forming elements such as chromium, molybdenum, aluminum and vanadium can be treated to produce hard surface layers providing improved wear resistance. The nitrides formed are not only hard but also more voluminous than the original steel and therefore they create compressive residual surface stresses which offer both improved corrosion and fatigue resistance.
Passive Films: Austenitic stainless steels and hardenable stainless steels such as martensitic, precipitation hardening and maraging stainless steels are seldom coated but their corrosion resistance depends on the formation of naturally occurring transparent oxide films. The production of these films is inhibited by surface contaminants and treatments are available to clean and degrease surfaces to help produce uniform protective oxide films under controlled conditions.
Metallic Coatings
Metallic coatings are deposited by electroplating, electroless plating, spraying, hot dipping, chemical vapor deposition and ion vapor deposition. The most common coatings used in aircraft are cadmium, chromium, nickel, aluminum and zinc.
Cadmium: Cadmium is widely used as an electroplating on steel fasteners however it is being phased out due to the toxicity of both the product and the process. Cadmium is anodic to steel and will cathodically protect the substrate at scratches or gaps in the coating at cut edges. It also exhibits surface lubricity, conductivity, resist fretting and. Coatings are usually 5 to 25 µm thick.
Chromium: Chromium is used on steel as a protective coating providing resistance to wear, abrasion and corrosion. It has a hardness in the range 900 to 1100HV, low friction characteristics and high reflectivity. It is used as a thin coating usually in the range 0.2 to 1µm thick as the final layer in a multiple-plate copper-nickel-chromium electroplating, or as a thick coating up to 300µm to provide wear resistance. The corrosion resistance is derived primarily from the barrier effect of the thick nickel plate of the chromium.Chromium is a metal with lower cathode efficient and substantial amounts of hydrogen are deposited on the part along with the metal being plated. Because of this, parts must be baked as soon as possible after plating to drive off the hydrogen and prevent embrittlement. Baking temperatures can be used up to the tempering temperature of the steel and therefore they tend to be less effective for very high strength steels where tempering temperatures are low.
Aluminum: Aluminum coatings can be applied to steel by hot dipping, cementation, ion vapor deposition or spraying. Spraying is the only process that has been used extensively over a long period of time with airframe parts. Pack cementation is widely used for turbine engine components.Sprayed aluminum coatings are around 100 to 150µm thick. Ion vapor coatings can be applied down to a minimum of 8 to 25 µm.
Zinc: Zinc coatings may be applied either by electroplating or spraying. Electroplatings are normally less than 25 µmthick and may be as thin as 5 µm on threaded parts. However, while they provide good protection to steel in rural atmospheres, they do not perform as well in marine or industrial environments. Zinc plating does not perform as well as cadmium in tropical and marine atmospheres, and therefore cadmium is preferred for aircraft use.
